With an increasing industry-wide focus on patient-centricity, there is a shift toward greater clinical trial (CT) transparency. Clinical trial summaries (CTSs) are a natural extension of this effort and will become a requirement in the EU with the Clinical Trial Regulation (Regulation (EU) No 536/2014) going live on 31 January 2022.
What are clinical trial summaries (CTSs)?
A CTS is a summary of interventional CT results, intended for anyone without a medical background. CTSs are primarily created to educate and inform study participants about the trial in which they participated.
Whom do CTSs benefit?
While CTSs are obviously useful to patients who participated in that specific clinical study, they also benefit other patients with the medical condition studied in the trial, caregivers, family members, patient advocacy groups (PAGs), healthcare professionals (HCPs), allied healthcare workers, other researchers, the media, and the general public.
However, for CTSs to be effective educational tools, they need to be relevant, accessible, and easy to understand without oversimplifying information. Health literacy plays a crucial role here, and what is worrisome is that most countries* have low health literacy levels. Health literacy is defined as “the personal characteristics and social resources needed for individuals and communities to access, understand, appraise and use information and services to make decisions about health.”
In the US, more than a third of the adult population experiences difficulty with common health tasks, such as understanding instructions on a prescription drug label or following a childhood immunization schedule with the help of a standard chart. In England, between 43% and 61% of working age adults do not routinely comprehend health information.
Such lack of understanding can lead to misinterpretations while reading medical information and adverse health effects, which can go on to create public mistrust in pharma. Let’s see how CTSs can be useful in this scenario with the help of some solid facts.
Fact check!
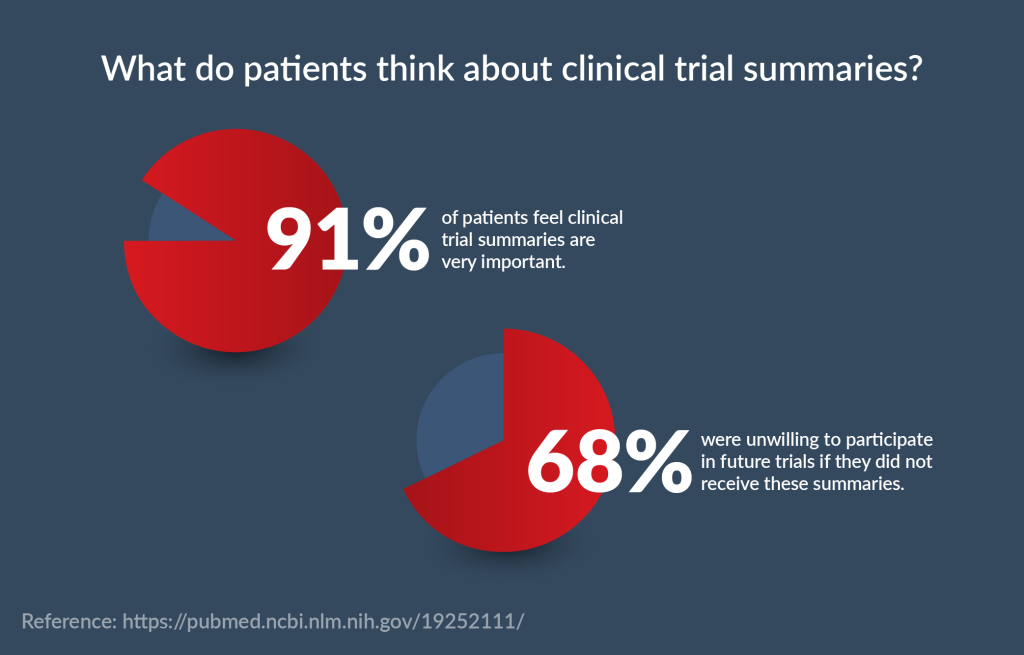
CTSs are the best approach for patient recruitment and retention. It is estimated that over 50% of the top pharma companies (including Johnson & Johnson, Roche, Pfizer, Novartis, etc.) use them. In fact, a study indicated that 91% of patients feel that study summaries are very important, and 68% were unwilling to participate in future trials if they did not receive these summaries. The results of another survey, by the Center for Information and Study on Clinical Research Participation, resonate with these findings.
How can CTSs help pharma build public trust?
There are several barriers hindering patient participation in CTs. Some of them are as follows: the lack of awareness that CTs can be a potential treatment option, confusion and fear regarding trial research and protocol, lack of trial-related information, and limited access to trial summaries and personal trial data.
CTSs make clinical study results more transparent, thereby promoting public trust, partnership, and patient engagement. Such summaries make research findings accessible to the public by peeling them off from behind journal paywalls and doing away with the need to navigate scientific writing’s often impenetrable communication style. They also make research findings easier to be translated into different languages.
How can PAGs and patient networks help pharma?
PAGs and patient networks play a crucial role in clinical study recruitment. Many of them have an active social media presence (e.g. @ChronicUTIAus, @NPAF_tweets, @pagthals, etc.) with their own websites and discussion boards. Pharma study sponsors and contract research organizations can associate with these groups and networks to inform patients about new CTs, including recruitment, trial protocol design, study progress, and outcomes.
For example, Biogen was able to scale up from screening only 6 patients/week to screening approximately 400 patients/week via the Biogen IDEC–MyHealthTeams partnership for recruitment in CTs. Similarly, pharma can use CTSs to build stronger relationships with PAGs as CTSs build a comprehensive picture of clinical study findings and promote patient empowerment.
How could CTSs have helped during COVID-19?
Many of the SARS-CoV-2 studies registered on ClinicalTrials.gov will probably be of no use to mitigating the COVID-19 pandemic as they have overlapping methodologies, lack synergy, or will be impossible to complete due to fluctuating numbers of study participants. The “infodemic” and misinformation spread via various media channels because of such trials have serious consequences. Examples of such misinformation include the changing perspective of chloroquine/hydroxychloroquine efficacy and toxicity and inflated public trust in remdesivir’s benefits.
Had there been well-drafted CTSs, with clearer and easy-to-understand information, made publicly available for the SARS-CoV-2 CTs, public mistrust in research/pharma could have been much lower.
CTSs can help prevent the dissemination of incorrect, confusing, and misleading information by directly talking to the patients. The EU CTR 536/2014 will help with this by providing timely access to CTSs of all the CTs conducted in the EU, via a dedicated portal and a database.
How will pharma benefit from the EU CTR 536/2014?
The goal of the EU CTR 536/2014 is to provide a favorable environment in the EU for conducting CTs, with high-quality and consistent safety standards and greater transparency. This regulation will have many benefits, including
- harmonizing the electronic submission and assessment process for CTs
- increasing the accessibility and visibility of CTSs
- increasing research literacy and participation
- augmenting patient engagement and increasing patient trust in pharma
- accelerating and augmenting drug development
- and stripping away administrative red-tape
In the end, the ultimate goal of creating CTSs is to speak the same language as the patients and be compassionate so that they feel heard and comfortable when talking to pharma and pharma can talk to patients more “compliantly”.
(*Certain sources that were cited here contain links that are now dead and have hence been removed from this article.)

Kwisha Shah
Marketing Content Manager


Thank you for the information. Very well detailed and explained. Excellent blog.