The COVID-19 pandemic has brought with it a deluge of preprints. While timely sharing of knowledge has helped us in forming an efficient response to control the ongoing crisis, how do we strike a balance between open sharing of information and reducing the confusion generated by information overload? I attended a debate session at the Virtual 17th Annual ISMPP Meeting to delve into this topic. Moderated by Lisa Jolly, Senior Scientific Specialist, Parexel, the session was titled A preprint pandemic: the evolving role of preprint servers in the COVID-19 era & Preprint debate – to preprint or not?, and had an impressive line-up of speakers, namely, Richard Sever, Assistant Director, Cold Spring Harbor Laboratory Press; Joanne Walker, Future Medicine; Tim Koder, Communications Director, Oxford PharmaGenesis; Meghan Johnson-Watson, Global Publications Leader, Oncology, AstraZeneca; and Janet Davies, Global Publications Lead, UCB Pharma.
Below is a summary of all that was discussed.
Preprints are unpublished manuscripts yet to be certified by peer review. bioRxiv was launched in 2013 as a preprint repository for the biological sciences and currently hosts 120,000 papers. medRxiv was launched in mid-2019 to meet the enhanced screening and more rigorous ethical requirements for dissemination of clinical information and hosts >16,000 medical preprints. Both repositories are non-profit and offer free submission and access.
In this session, speakers representing the publisher, the pharmaceutical industry, and the preprint server shared their perspectives on the evolving role of preprints. This was followed by a debate in support of and against the use of preprints for the dissemination of clinical trial results.
Publisher perspective
A significant increase in preprints, including both Covid-19 and non-Covid-19 publications, has been observed over the past few years. Advantages of preprints include swift access to new research findings, an open review model, and insights into ongoing research.
During the pandemic, STM publishers encouraged authors to post their manuscripts on preprint servers, which is not considered prior publication. However, authors may be reluctant to do so since journals lack a consistent policy on preprints.
Preprints facilitate an open, collaborative peer review process; however, <10% of preprints receive comments. Authors may consider rapid peer review options offered by journals to avoid publication delays.
Industry perspective
Publication timelines were stretched during the pandemic because of delays in receiving author comments or feedback from journal reviewers. This led to limited opportunities for rapid review of publications. Congress deadlines were extended, and little time was available between abstract acceptance notification and the congress presentation.
Preprints may be an attractive option for data dissemination given the challenges associated with timely publication during the pandemic; however, only certain types of data, for example, academic research data or preclinical data not related to investigational products, seem suitable for preprints. Clinical data are unsuitable for posting as preprints because the absence of peer review may lead to compromised data integrity and inaccuracies in reporting. There are also concerns about data misinterpretation as well as off-label misuse.
Considerations around the use of preprints

Preprint server perspective
Rapid communication is critical during pandemics. In 2003, 93% of SARS-related papers were published after the epidemic was controlled. In contrast, ~14,000 preprints were available in 2020 during the ongoing pandemic. Some preprints accelerated the dissemination of critical information as evidenced by their high usage statistics.
Example of a highly accessed preprint
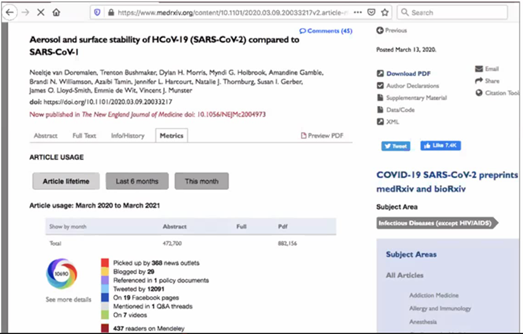
Covid-19-related preprints provided access to early epidemiological observations, immunological response, virus structure and biology, data from observational studies of standard-of-care treatment, and clinical trial results of hydroxychloroquine, tocilizumab, convalescent plasma, and dexamethasone.
During the pandemic, bioRxiv and medRxiv preprints are being screened by PhDs or MDs for general criteria such as plagiarism, concerns around compromising patient identity, and adhering to ethical standards as well as the overall principle of “do no harm,” for example, dual-use concerns, papers challenging vaccine safety and public health measures, or those propagating conspiracy theories or concerns around drug availability.
In the future, traditional journal peer reviews may be augmented by portable peer review platforms, commenting platforms, and services offering automated analyses of content.
Alternative venues for rapid peer review of Covid-19-related literature posted on preprint archives

Summary of the debate representing views in favor of and against the use of preprints of clinical trial papers

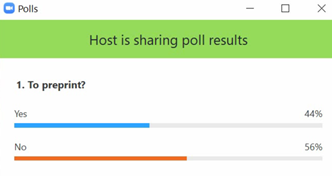
Result of audience poll after presentation
This article is a part of the report on the 17th Virtual Annual Meeting of ISMPP. Click here to get your copy of the comprehensive report today.

Sandeep Kamat
Sandeep Kamat is Vice President, Quality & Training, at Cactus Life Sciences.

